The covid-19 vaccine patent application of the team of academician Chen Wei has been authorized
New progress in scientific research brings the new hopes for life. On August 11, the National Intellectual Property Administration issued a patent authorization notice to the applicants, the Military Medical Research Institute of the Academy of Military Sciences of the Chinese People’s Liberation Army and Kansino Biological Co., Ltd., granting patent right to the patent application for invention called “A recombinant new coronavirus vaccine using human replication-deficient adenovirus as a vector” (application number: 202010193587.8), which strengthens the faith for people to win the battle against epidemic.
One of the inventors of this patent for invention is Academician Chen Wei, a researcher at the Academy of Military Medicine of the Academy of Military Sciences who has just been awarded the national honorary title "People's Hero". When the epidemic outbroke, she was ordered to go to Wuhan immediately to carry out scientific research and epidemic prevention and control tasks. The vaccine patent application was granted patent right, which is one of the important achievements of her and her team. Chen Wei's team achieved phased results within just over 20 days since January 26 when they went to Wuhan to carry out vaccine research and development, and submitted a Chinese patent application on March 18. This is the “China speed” that builds the line of defense for health and life.
How was the efficient examination of this vaccine patent achieved? According to the "Administrative Measures for Patent Priority Examination" issued by the National Intellectual Property Administration (National Intellectual Property Administration Order No. 76), patent applications that are of great significance to the national or public interest and require priority examination can request priority examination. On February 15, the State Administration for Market Regulation, the State Food and Drug Administration, and the National Intellectual Property Administration issued the "Ten Articles on Resumption of Work and Production" which states that patent applications and trademark registrations related to the prevention and treatment of new coronary pneumonia should be examined in priority. According to a staff from the National Intellectual Property Administration, the patent applicant requested a priority examination in accordance with the "Administrative Measures for Patent Priority Examination" and the "Ten Articles on Resumption of Work and Production”. The National Intellectual Property Administration considered that it meets the priority examination standard and handles it according to the priority examination procedure. At the same time, the National Intellectual Property Administration treats all patent applications equally, controls the examination and authorization strictly, strengthens quality evaluation, and examines in accordance with the law strictly to ensure the authorization quality of priority examination patent applications.
The conditions to apply for priority examination
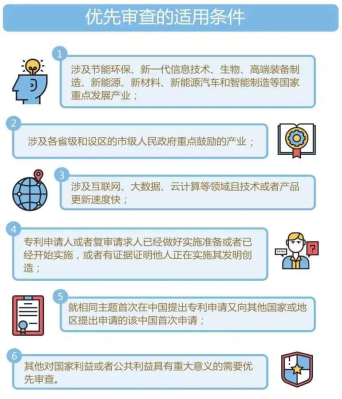
1. For industries that the country places emphasis on to develop such as energy conservation and environmental protection, new generation information technology, biology, high-end equipment manufacturing, new energy, new materials, new energy vehicles and smart manufacturing;
2. For industries that are mainly encouraged by the provincial governments and municipal governments which have districts;
3. For industries that are related to the Internet, big data, cloud computing and other fields and the update speed of such technology or product is fast;
4. The patent applicant or reexamination applicant is ready for implementation or has begun implementation, or there is evidence that others are implementing the invention;
5. The application is the first Chinese application that is also applied to another country or region for the same subject;
6. Others that are of great significance to the national or public interest and require priority examination
The procedure of priority examination
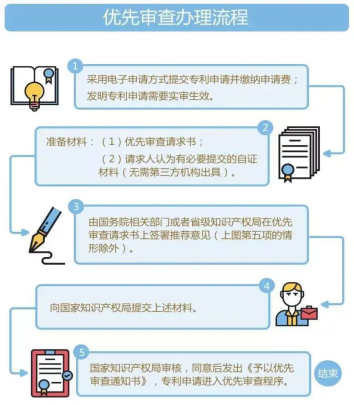
1. Submit patent applications by electronic application and pay application fees; patent applications for invention need to be validated upon real examination;
2. Prepare Materials: (1) Priority examination request; (2) Self-certification materials that the applicants consider necessary (no need to be issued by a third-party institution);
3. The relevant department of the State Council or the provincial intellectual property office sign the recommendation opinion on the priority examination request (except for the situation in item 5 in the above picture);
4. Submit the above-mentioned materials;
5. The National Intellectual Property Administration examines and issues a "Notice of Priority Examination" after approval, and the patent application enters the priority examination procedure.
"Overcoming the epidemic is inseparable from the support of science and technology." At the forefront of the battle against the epidemic, there are also a large number of scientific research workers like Chen Wei. They fought courageously day and night, and produced a large number of key original scientific research results. In order to fully support the prevention and control of the epidemic, the National Intellectual Property Administration has dealt with various patent applications or cases related to the prevention and treatment of new coronary pneumonia, such as drugs used to prevent or treat the new coronary pneumonia, vaccines, testing methods, protective products and methods like masks, goggles, and disinfectants. National Intellectual Property Administration implements the above-mentioned policies strictly, carries out priority examination request equally, and has strongly supported the prevention and control of the epidemic and the resumption of production.
As special products for healthy people, vaccines are required to meet higher standards for safety and effectiveness. Relevant experts pointed out that the patent right granted to the new coronavirus vaccine is only a preliminary result. There is still a long way to go from patents to mature products that can be truly used for epidemic prevention and control. “Vaccines are related to life and health and require rigorous clinical trials and strict approval by relevant departments before they can be truly marketed and put into clinical applications. Enterprises and scientific research institutions must continue to promote development and industrialization of the vaccine on the basis of ensuring safety and effectiveness. We will go all out to win the battle against the epidemic.” said Tao Xinliang, honorary dean of the School of Intellectual Property, University of Dalian Technology.
"As long as we strengthen our faith, work together in the same boat, prevent and control scientifically, and implement precise policies, we will definitely win the battle against epidemic." From scientific research to patent examination to clinical application, we together fight the "epidemic" with one heart. We will definitely witness the double victory of epidemic control and economic and social development.
Source:中国知识产权报
Author:孙迪
Translated by CIP Lawyers(www.ciplawyer.com)
-
Previous:
-
Next: